1
Harumi, OkuyamaPeter H, LangsjoenTomohito, HamazakiYoichi, OgushiRokuro, HamaTetsuyuki, KobayashiHajime, Uchino. (2015) Statins stimulate atherosclerosis and heart failure: pharmacological mechanisms. Expert Review of Clinical Pharmacology 8, 189-199
CrossRef
2
Huifeng, RenZehuan, HuangHui, YangHuaping, XuXi, Zhang. (2015) Controlling the Reactivity of the SeSe Bond by the Supramolecular Chemistry of Cucurbituril. ChemPhysChem 16:10.1002/cphc.v16.3, 523-527
CrossRef
3
P., VatsN., SagarT. P., SinghM., Banerjee. (2015) Association of
Superoxide dismutases
(SOD1 and SOD2) and
Glutathione peroxidase 1 (GPx1)
gene polymorphisms with Type 2 diabetes mellitus. Free Radical Research 49, 17-24
CrossRef
4
Judy B., de Haan. (2014) Limiting reductive stress for treating in-stent stenosis: the heart of the matter?. Journal of Clinical Investigation 124, 5092-5094
CrossRef
5
Ziad A., AliVinicio, de Jesus PerezKe, YuanMark, OrcholskiStephen, PanWei, QiGaurav, ChopraChristopher, AdamsYoko, KojimaNicholas J., LeeperXiumei, QuKathia, Zaleta-RiveraKimihiko, KatoYoshiji, YamadaMitsutoshi, OguriAllan, KuchinskyStanley L., HazenJ. Wouter, JukemaSanthi K., GaneshElizabeth G., NabelKeith, ChannonMartin B., LeonAlain, CharestThomas, QuertermousEuan A., Ashley. (2014) Oxido-reductive regulation of vascular remodeling by receptor tyrosine kinase ROS1. Journal of Clinical Investigation 124, 5159-5174
CrossRef
6
Mohammad, NajafiHassan, GhasemiAbazar, RoustazadehMohammad, Farajollahi. (2014) Lack of association between glutathione peroxidase1 (GPx1) activity, Pro198Leu polymorphism and stenosis of coronary arteries: A population-based prediction. Meta Gene 2, 722-729
CrossRef
7
Tanjina, KaderCarolyn M., PorteousMichael J.A., WilliamsSteven P., GiesegSally P.A., McCormick. (2014) Ribose-cysteine increases glutathione-based antioxidant status and reduces LDL in human lipoprotein(a) mice. Atherosclerosis 237, 725-733
CrossRef
8
S., YangM. K., JensenE. B., RimmW., WillettT., Wu. (2014) Erythrocyte Superoxide Dismutase, Glutathione Peroxidase, and Catalase Activities and Risk of Coronary Heart Disease in Generally Healthy Women: A Prospective Study. American Journal of Epidemiology 180, 901-908
CrossRef
9
Swathi, SeshadriStephanie, MroczkowskaLu, QinSunni, PatelAniko, EkartDoina, Gherghel. (2014) Systemic circulatory influences on retinal microvascular function in middle-age individuals with low to moderate cardiovascular risk. Acta Ophthalmologica, n/a-n/a
CrossRef
10
Huige, LiSven, HorkeUlrich, Förstermann. (2014) Vascular oxidative stress, nitric oxide and atherosclerosis. Atherosclerosis 237, 208-219
CrossRef
11
Livan, Delgado-RocheJulio R., FernándezDalia R., Álvarez. (2014) Glutathione peroxidase-1 expression is up-regulated by ozone therapy in ApoE deficient mice. Biomedicine & Aging Pathology 4, 323-326
CrossRef
12
Mariana Appel, HortMarcos Raniel, StraliottoJade, de OliveiraNívea Dias, AmoêdoJoão Batista Teixeira, da RochaAntônio, GalinaRosa Maria, Ribeiro-do-ValleAndreza Fabro, de Bem. (2014) Diphenyl diselenide protects endothelial cells against oxidized low density lipoprotein-induced injury: Involvement of mitochondrial function. Biochimie 105, 172-181
CrossRef
13
Peramaiyan, RajendranNatarajan, NandakumarThamaraiselvan, RengarajanRajendran, PalaniswamiEdwinoliver Nesamony, GnanadhasUppalapati, LakshminarasaiahJacob, GopasIkuo, Nishigaki. (2014) Antioxidants and human diseases. Clinica Chimica Acta 436, 332-347
CrossRef
14
A, MoslehiM, Taghizadeh-GhehiK, GholamiM, HadjibabaieZ, Jahangard-RafsanjaniA, SarayaniM, JavadiM, EsfandbodA, Ghavamzadeh. (2014) N-acetyl cysteine for prevention of oral mucositis in hematopoietic SCT: a double-blind, randomized, placebo-controlled trial. Bone Marrow Transplantation 49, 818-823
CrossRef
15
Loscalzo , Joseph . (2014) Keshan Disease, Selenium Deficiency, and the Selenoproteome. New England Journal of Medicine 370:18, 1756-1760
Full Text
16
Temidayo O., OmobowaleAdemola A., OyagbemiAkinleye S., AkinrindeAdebowale B., SabaOluwabusola T., DaramolaBlessing S., OgunpoluJames O., Olopade. (2014) Failure of recovery from lead induced hepatoxicity and disruption of erythrocyte antioxidant defence system in Wistar rats. Environmental Toxicology and Pharmacology 37, 1202-1211
CrossRef
17
Elena V., SuprunVictoria V., ShumyantsevaAlexander I., Archakov. (2014) Protein Electrochemistry: Application in Medicine. A Review. Electrochimica Acta
CrossRef
18
Carlota, SaldanhaJosé, de AlmeidaAna, Silva-Herdade. (2014) Application of a Nitric Oxide Sensor in Biomedicine. Biosensors 4, 1-17
CrossRef
19
Salah A., SheweitaHeba A., El-BenderyMostafa H., Mostafa. (2014) Novel Study on N-Nitrosamines as Risk Factors of Cardiovascular Diseases. BioMed Research International 2014, 1-10
CrossRef
20
G., SilbernagelB., SchottkerS., AppelbaumH., ScharnaglM. E., KleberT. B., GrammerA., RitschU., MonsB., HolleczekG., GoliaschA., NiessnerB. O., BoehmR. B., SchnabelH., BrennerS., BlankenbergU., LandmesserW., Marz. (2013) High-density lipoprotein cholesterol, coronary artery disease, and cardiovascular mortality. European Heart Journal 34, 3563-3571
CrossRef
21
Gianni, ManciniJade, de OliveiraMariana Appel, HortEduardo Luiz Gasnhar, MoreiraRosa Maria, Ribeiro-do-ValleJoão Batista Texeira, RochaAndreza Fabro, de Bem. (2013) Diphenyl diselenide differently modulates cardiovascular redox responses in young adult and middle-aged low-density lipoprotein receptor knockout hypercholesterolemic mice. Journal of Pharmacy and Pharmacology, n/a-n/a
CrossRef
22
Monisha, BanerjeePushpank, Vats. (2013) Reactive metabolites and antioxidant gene polymorphisms in Type 2 Diabetes Mellitus. Redox Biology
CrossRef
23
Karen, ReesLouise, HartleyNadine, FlowersAileen, ClarkeLee, HooperMargaret, ThorogoodSaverio, StrangesKaren, Rees. 'Mediterranean' dietary pattern for the primary prevention of cardiovascular disease. 2013.
CrossRef
24
Lutz, SchomburgJosef, Köhrle. Selen. 2013:, 243-286.
CrossRef
25
David, CoquerelEva, KušíkováPaul, MulderMoïse, CoëffierSylvanie, RenetPierre, DechelotteVincent, RichardChristian, ThuillezFabienne, Tamion. (2013) Omega-3 Polyunsaturated Fatty Acids Delay the Progression of Endotoxic Shock-Induced Myocardial Dysfunction. Inflammation 36, 932-940
CrossRef
26
Nageswara R., MadamanchiMarschall S., Runge. (2013) Redox signaling in cardiovascular health and disease. Free Radical Biology and Medicine 61, 473-501
CrossRef
27
Marcos Raniel, StraliottoMariana Appel, HortBianca, FiuzaJoão Batista Teixeira, RochaMarcelo, FarinaGustavo, ChiabrandoAndreza Fabro, de Bem. (2013) Diphenyl diselenide modulates oxLDL-induced cytotoxicity in macrophage by improving the redox signaling. Biochimie 95, 1544-1551
CrossRef
28
Serap Gunes, BilgiliHalil, OzkolZennure, TakciHatice Uce, OzkolAyse Serap, KaradagMehmet, Aslan. (2013) Assessment of the serum paraoxonase activity and oxidant/antioxidant status in patients with recurrent aphthous stomatitis. International Journal of Dermatology, n/a-n/a
CrossRef
29
Kausik, ChatterjeeKazi Monjur, AliDebasis, DeTushar Kanti, BeraKishalay, JanaSoumyajit, MaitiAbhinandan, GhoshDebidas, Ghosh. (2013) Hyperglycemia-induced alteration in reproductive profile and its amelioration by the polyherbal formulation MTEC (modified) in streptozotocin-induced diabetic albino rats. Genomic Medicine, Biomarkers, and Health Sciences
CrossRef
30
Huige, LiSven, HorkeUlrich, Förstermann. (2013) Oxidative stress in vascular disease and its pharmacological prevention. Trends in Pharmacological Sciences 34, 313-319
CrossRef
31
Dong Hoon, KangSang Won, Kang. (2013) Targeting Cellular Antioxidant Enzymes for Treating Atherosclerotic Vascular Disease. Biomolecules and Therapeutics 21, 89-96
CrossRef
32
Nitin T., AggarwalJonathan C., Makielski. (2013) Redox Control of Cardiac Excitability. Antioxidants & Redox Signaling 18, 432-468
CrossRef
33
Fairuz, RasdiNor, BakarSharifah, Mohamad. (2013) A Comparative Study of Selected Trace Element Content in Malay and Chinese Traditional Herbal Medicine (THM) Using an Inductively Coupled Plasma-Mass Spectrometer (ICP-MS). International Journal of Molecular Sciences 14, 3078-3093
CrossRef
34
Marcos Raniel, StraliottoJade de, OliveiraGianni, ManciniAfonso C.D., BainyAlexandra, LatiniAnna Maria, DeobaldJoão B.T., RochaAndreza Fabro de, Bem. (2013) Disubstituted diaryl diselenides as potential atheroprotective compounds: involvement of TrxR and GPx-like systems. European Journal of Pharmaceutical Sciences
CrossRef
35
Claire M., WeekleyHugh H., Harris. (2013) Which form is that? The importance of selenium speciation and metabolism in the prevention and treatment of disease. Chemical Society Reviews 42, 8870
CrossRef
36
Edwin, HoKeyvan, Karimi GalougahiChia-Chi, LiuRavi, BhindiGemma A., Figtree. (2013) Biological markers of oxidative stress: Applications to cardiovascular research and practice. Redox Biology 1, 483-491
CrossRef
37
Junrui, PeiWenqi, FuLiu, YangZhiyi, ZhangYang, Liu. (2013) Oxidative Stress is Involved in the Pathogenesis of Keshan Disease (an Endemic Dilated Cardiomyopathy) in China. Oxidative Medicine and Cellular Longevity 2013, 1-5
CrossRef
38
Elisângela, ColpoCarlos Dalton de Avila, VilanovaLuiz Gustavo, Brenner ReetzMarta Maria, Medeiros Frescura DuarteIria Luiza Gomes, FariasEdson, Irineu MullerAline Lima Hermes, MullerErico Marlon, Moraes FloresRoger, WagnerJoão Batista Teixeira, da Rocha. (2013) A Single Consumption of High Amounts of the Brazil Nuts Improves Lipid Profile of Healthy Volunteers. Journal of Nutrition and Metabolism 2013, 1-7
CrossRef
39
Domenico, LapennaGiuliano, CiofaniChiara, CuccurulloSante Donato, PierdomenicoFranco, Cuccurullo. (2012) Ascorbic acid supplementation reduces oxidative stress and platelet biochemical function in type 2 diabetic patients. Relevance of ascorbic acid dosage and formulation. e-SPEN Journal 7, e245-e248
CrossRef
40
Christian, Weber. Neutrophilic Granulocytes in the Treatment of Atherosclerosis. 2012:, 365-391.
CrossRef
41
Yu. A., ShuvalovaA. I., KaminnyiA. N., MeshkovR. O., ShirokovA. N., Samko. (2012) Association between polymorphisms of eNOS and GPx-1 genes, activity of free-radical processes and in-stent restenosis. Molecular and Cellular Biochemistry 370, 241-249
CrossRef
42
Joanna, Śladowska-KozłowskaMieczysław, LitwinAnna, NiemirskaPaweł, PłudowskiAldona, WierzbickaEwa, SkorupaZbigniew T., WawerRoman, Janas. (2012) Oxidative stress in hypertensive children before and after 1 year of antihypertensive therapy. Pediatric Nephrology 27, 1943-1951
CrossRef
43
A., O'Connor. (2012) An overview of the role of bread in the UK diet. Nutrition Bulletin 37:10.1111/nbu.2012.37.issue-3, 193-212
CrossRef
44
Oxidative Stress. 2012:, 120-138.
CrossRef
45
Biljana, MusickiTongyun, LiuSena F., SezenArthur L., Burnett. (2012) Targeting NADPH Oxidase Decreases Oxidative Stress in the Transgenic Sickle Cell Mouse Penis. The Journal of Sexual Medicine 9:10.1111/jsm.2012.9.issue-8, 1980-1987
CrossRef
46
Maria E., LönnJoanne M., DennisRoland, Stocker. (2012) Actions of “antioxidants” in the protection against atherosclerosis. Free Radical Biology and Medicine 53, 863-884
CrossRef
47
Heiner K., BertholdBernhard, MichalkeWilhelm, KroneEliseo, GuallarIoanna, Gouni-Berthold. (2012) Influence of serum selenium concentrations on hypertension. Journal of Hypertension 30, 1328-1335
CrossRef
48
Brunna Cristina Bremer, BoaventuraPatrícia Faria, Di PietroAliny, StefanutoGraziela Alessandra, KleinElayne Cristina, de MoraisFernanda, de AndradeElisabeth, WazlawikEdson Luiz, da Silva. (2012) Association of mate tea (Ilex paraguariensis) intake and dietary intervention and effects on oxidative stress biomarkers of dyslipidemic subjects. Nutrition 28, 657-664
CrossRef
49
T. V., ZheikovaM. V., GolubenkoS. V., BuikinO. Yu., BotkinaO. A., MakeevaA. A., LezhnevE. V., KalyanovI. V., TsimbalyukV. N., MaksimovM. I., VoevodaV. M., ShipulinV. P., Puzyrev. (2012) Glutathione peroxidase 1 (GPX1) single nucleotide polymorphism Pro198→Leu: Association with life span and coronary artery disease. Molecular Biology 46, 433-437
CrossRef
50
Karen, ReesLouise, HartleyAileen, ClarkeMargaret, ThorogoodSaverio, StrangesKaren, Rees. 'Mediterranean' dietary pattern for the primary prevention of cardiovascular disease. 2012.
CrossRef
51
Nidhi, ChaudharyKiran Kumar, NakkaNilanjana, MaulikSamit, Chattopadhyay. (2012) Epigenetic Manifestation of Metabolic Syndrome and Dietary Management. Antioxidants & Redox Signaling, 120302065839003
CrossRef
52
Christine, KoulisJudy B, de HaanTerri J, Allen. (2012) Novel pathways and therapies in experimental diabetic atherosclerosis. Expert Review of Cardiovascular Therapy 10, 323-335
CrossRef
53
Elena V., SuprunAnatoly A., SavelievGennady A., EvtugynAlexander V., LisitsaTatiana V., BulkoVictoria V., ShumyantsevaAlexander I., Archakov. (2012) Electrochemical approach for acute myocardial infarction diagnosis based on direct antibodies-free analysis of human blood plasma. Biosensors and Bioelectronics 33, 158-164
CrossRef
54
Margaret P, Rayman. (2012) Selenium and human health. The Lancet 379, 1256-1268
CrossRef
55
Diane E., HandyJoseph, Loscalzo. (2012) Redox Regulation of Mitochondrial Function. Antioxidants & Redox Signaling, 120203070121007
CrossRef
56
Tsz-Shan, KamCho-Yee, WongPui-Long, KwanWing, Fat-YiuSin-Ming, ChiuShun-Wan, ChanKit-San, YuenRobbie, Chan. (2012) Effects and Mechanism of Turmeric Vasorelaxation of the Thoracic Aorta in Hypercholesterolemic Rats. Journal of Medicinal Food 15, 190-199
CrossRef
57
T.S., TangS.L., PriorK.W., LiH.A., IrelandS.C., BainS.J., HurelJ.A., CooperS.E., HumphriesJ.W., Stephens. (2012) Association between the rs1050450 glutathione peroxidase-1 (C��>��T) gene variant and peripheral neuropathy in two independent samples of subjects with diabetes mellitus. Nutrition, Metabolism and Cardiovascular Diseases 22:5, 417
CrossRef
58
Wei, YangJuan, FuMiao, YuQingde, HuangDi, WangJiqu, XuQianchun, DengPing, YaoFenghong, HuangLiegang, Liu. (2012) Effects of flaxseed oil on anti-oxidative system and membrane deformation of human peripheral blood erythrocytes in high glucose level. Lipids in Health and Disease 11, 88
CrossRef
59
Arpeeta, SharmaPascal N., BernatchezJudy B., de Haan. (2012) Targeting Endothelial Dysfunction in Vascular Complications Associated with Diabetes. International Journal of Vascular Medicine 2012, 1-12
CrossRef
60
Karen, ReesLouise, HartleyAileen, ClarkeMargaret, ThorogoodSaverio, StrangesKaren, Rees. Cochrane Database of Systematic Reviews Protocols. 2012.
CrossRef
61
J. L., Martin-VenturaJ., Madrigal-MatuteR., Martinez-PinnaP., Ramos-MozoL. M., Blanco-ColioJ. A., MorenoC., TarinE., BurilloC. E., Fernandez-GarciaJ., EgidoO., MeilhacJ.-B., Michel. (2012) Erythrocytes, leukocytes and platelets as a source of oxidative stress in chronic vascular diseases: Detoxifying mechanisms and potential therapeutic options. Thrombosis and Haemostasis 108
CrossRef
62
Deniz Cemgil, ArikanAyhan, CoskunAli, OzerMetin, KilincFiliz, AtalayTugba, Arikan. (2011) Plasma Selenium, Zinc, Copper and Lipid Levels in Postmenopausal Turkish Women and Their Relation with Osteoporosis. Biological Trace Element Research 144, 407-417
CrossRef
63
Maysam, TakapooAli H., ChamseddineRamesh C., BhallaFrancis J., Miller. (2011) Glutathione peroxidase-deficient smooth muscle cells cause paracrine activation of normal smooth muscle cells via cyclophilin A. Vascular Pharmacology 55, 143-148
CrossRef
64
Tharmarajan, RamprasathPonniah Senthil, MuruganEllappan, KalaiarasanPannerselvam, GomathiAndiappan, RathinavelGovindan Sadasivam, Selvam. (2011) Genetic association of Glutathione peroxidase-1 (GPx-1) and NAD(P)H:Quinone Oxidoreductase 1(NQO1) variants and their association of CAD in patients with type-2 diabetes. Molecular and Cellular Biochemistry
CrossRef
65
Edith, LubosJoseph, LoscalzoDiane E., Handy. (2011) Glutathione Peroxidase-1 in Health and Disease: From Molecular Mechanisms to Therapeutic Opportunities. Antioxidants & Redox Signaling 15, 1957-1997
CrossRef
66
Koichi, SugamuraJohn F., Keaney,. (2011) Reactive oxygen species in cardiovascular disease. Free Radical Biology and Medicine 51, 978-992
CrossRef
67
Jong-Gil, ParkGoo-Taeg, Oh. (2011) The role of peroxidases in the pathogenesis of atherosclerosis. BMB Reports 44, 497-505
CrossRef
68
Ulrich, SchweizerNora, DehinaLutz, Schomburg. (2011) Disorders of selenium metabolism and selenoprotein function. Current Opinion in Pediatrics 23, 429-435
CrossRef
69
Mariana Appel, HortMarcos Raniel, StraliottoPaula Moro, NettoJoão Batista Teixeira, da RochaAndreza Fabro, de BemRosa Maria, Ribeiro-do-Valle. (2011) Diphenyl Diselenide Effectively Reduces Atherosclerotic Lesions in LDLr −/− Mice by Attenuation of Oxidative Stress and Inflammation. Journal of Cardiovascular Pharmacology 58, 91-101
CrossRef
70
Saverio, StrangesFerruccio, GallettiEduardo, FarinaroLanfranco, D’EliaOrnella, RussoRoberto, IaconeClemente, CapassoVincenzo, CarginaleViviana, De LucaElisabetta, Della ValleFrancesco P., CappuccioPasquale, Strazzullo. (2011) Associations of selenium status with cardiometabolic risk factors: An 8-year follow-up analysis of the Olivetti Heart Study. Atherosclerosis 217, 274-278
CrossRef
71
Dan, FarbsteinYitzchak Z., SoloveichikNina S., LevyAndrew P., Levy. (2011) Genetics of Redox Systems and Their Relationship with Cardiovascular Disease. Current Atherosclerosis Reports 13, 215-224
CrossRef
72
Rosaria, VarìMassimo, D'ArchivioCarmelina, FilesiSimona, CarotenutoBeatrice, ScazzocchioCarmela, SantangeloClaudio, GiovanniniRoberta, Masella. (2011) Protocatechuic acid induces antioxidant/detoxifying enzyme expression through JNK-mediated Nrf2 activation in murine macrophages. The Journal of Nutritional Biochemistry 22, 409-417
CrossRef
73
Michael, MaesPiyanuj, RuckoanichYoung Seun, ChangNithi, MahanondaMichael, Berk. (2011) Multiple aberrations in shared inflammatory and oxidative & nitrosative stress (IO&NS) pathways explain the co-association of depression and cardiovascular disorder (CVD), and the increased risk for CVD and due mortality in depressed patients. Progress in Neuro-Psychopharmacology and Biological Psychiatry 35, 769-783
CrossRef
74
J. C., CharniotA., SuttonD., Bonnefont-RousselotC., CossonR., Khani-BittarP., GiralN., CharnauxJ. P., Albertini. (2011) Manganese superoxide dismutase dimorphism relationship with severity and prognosis in cardiogenic shock due to dilated cardiomyopathy. Free Radical Research 45, 379-388
CrossRef
75
Sunan, WangJohn P., MelnykRong, TsaoMassimo F., Marcone. (2011) How natural dietary antioxidants in fruits, vegetables and legumes promote vascular health. Food Research International 44, 14-22
CrossRef
76
Annateresa, PapazzoXavier, ConlanLouise, LexisPaul, Lewandowski. (2011) The effect of short-term canola oil ingestion on oxidative stress in the vasculature of stroke-prone spontaneously hypertensive rats. Lipids in Health and Disease 10, 180
CrossRef
77
Till, KellerStefan, Blankenberg. Novel biomarkers in evaluation of acute coronary syndrome. 2011:, 131-138.
CrossRef
78
Shin-ichiro, TanakaTetsuo, MikiShoto, ShaKen-ichi, HirataYuichi, IshikawaMitsuhiro, Yokoyama. (2011) Serum Levels of Thiobarbituric Acid-Reactive Substances are Associated with Risk of Coronary Heart Disease. Journal of Atherosclerosis and Thrombosis 18, 584-591
CrossRef
79
Yaling, YangSze Wa, ChanMiao, HuRichard, WaldenBrian, Tomlinson. (2011) Effects of Some Common Food Constituents on Cardiovascular Disease. ISRN Cardiology 2011, 1-16
CrossRef
80
Annateresa, PapazzoXavier A, ConlanLouise, LexisPaul A, Lewandowski. (2011) Differential effects of dietary canola and soybean oil intake on oxidative stress in stroke-prone spontaneously hypertensive rats. Lipids in Health and Disease 10, 98
CrossRef
81
S., StrangesA., Navas-AcienM.P., RaymanE., Guallar. (2010) Selenium status and cardiometabolic health: State of the evidence. Nutrition, Metabolism and Cardiovascular Diseases 20, 754-760
CrossRef
82
P., ChewD. Y. C., YuenN., StefanovicJ., PeteM. T., CoughlanK. A., Jandeleit-DahmM. C., ThomasF., RosenfeldtM. E., CooperJ. B. d., Haan. (2010) Antiatherosclerotic and Renoprotective Effects of Ebselen in the Diabetic Apolipoprotein E/GPx1-Double Knockout Mouse. Diabetes 59, 3198-3207
CrossRef
83
Sara, TucciSonja, PrimassinUte, Spiekerkoetter. (2010) Fasting-induced oxidative stress in very long chain acyl-CoA dehydrogenase-deficient mice. FEBS Journal 277, 4699-4708
CrossRef
84
Yu. A., ShuvalovaA. I., KaminnyiA. N., MeshkovV. V., Kukharchuk. (2010) Pro198Leu Polymorphism of GPx-1 Gene and Activity of Erythrocytic Glutathione Peroxidase and Lipid Peroxidation Products. Bulletin of Experimental Biology and Medicine 149, 743-745
CrossRef
85
S.G., DonaldsonJ., Van OostdamC., TikhonovM., FeeleyB., ArmstrongP., AyotteO., BoucherW., BowersL., ChanF., DallaireR., DallaireÉ., DewaillyJ., EdwardsG.M., EgelandJ., FontaineC., FurgalT., LeechE., LoringG., MuckleT., NancarrowD., PeregP., PlusquellecM., PotyralaO., ReceveurR.G., Shearer. (2010) Environmental contaminants and human health in the Canadian Arctic. Science of The Total Environment 408, 5165-5234
CrossRef
86
Robyn S., LymburyMatthew J., MarinoAnthony V., Perkins. (2010) Effect of dietary selenium on the progression of heart failure in the ageing spontaneously hypertensive rat. Molecular Nutrition & Food Research 54:10.1002/mnfr.v54:10, 1436-1444
CrossRef
87
Sayuri, MiyamotoHirofumi, AraiJunji, Terao. Enzymatic Antioxidant Defenses. 2010:, 21-33.
CrossRef
88
Eugene A, Podrez. (2010) Anti-oxidant properties of high-density lipoprotein and atherosclerosis. Clinical and Experimental Pharmacology and Physiology 37:10.1111/cep.2010.37.issue-7, 719-725
CrossRef
89
Cong, LeiXiaolin, NiuXiangke, MaJin, Wei. (2010) Is selenium deficiency really the cause of Keshan disease?. Environmental Geochemistry and Health
CrossRef
90
Chun-Seok, ChoSukmook, LeeGeun Taek, LeeHyun Ae, WooEui-Ju, ChoiSue Goo, Rhee. (2010) Irreversible Inactivation of Glutathione Peroxidase 1 and Reversible Inactivation of Peroxiredoxin II by H
2
O
2
in Red Blood Cells. Antioxidants & Redox Signaling 12, 1235-1246
CrossRef
91
I., GammoudiS., KhelilA., DandanaZ., JaidaneA., ChalghoumY., NoichriH., ChahedY., ChaabouniS., ErnezG., JeridiS., FerchichiA., Miled. (2010) Étude des marqueurs du stress oxydatif chez les coronariens tabagiques tunisiens. Immuno-analyse & Biologie Spécialisée 25, 135-139
CrossRef
92
Ulrich, Förstermann. (2010) Nitric oxide and oxidative stress in vascular disease. Pflügers Archiv - European Journal of Physiology 459, 923-939
CrossRef
93
Marcus, ConradUlrich, Schweizer. (2010) Unveiling the Molecular Mechanisms Behind Selenium-Related Diseases Through Knockout Mouse Studies. Antioxidants & Redox Signaling 12, 851-865
CrossRef
94
V. V., ShumyantsevaE. V., SuprunT. V., BulkoO. V., DobryninaA. I., Archakov. (2010) Sensor systems for medical application based on hemoproteins and nanocomposite materials. Biochemistry (Moscow) Supplement Series B: Biomedical Chemistry 4, 25-36
CrossRef
95
Edith, LubosChristoph R., SinningRenate B., SchnabelPhilipp S., WildTanja, ZellerHans J., RupprechtChristoph, BickelKarl J., LacknerDirk, PeetzJoseph, LoscalzoThomas, MünzelStefan, Blankenberg. (2010) Serum selenium and prognosis in cardiovascular disease: results from the AtheroGene study. Atherosclerosis 209, 271-277
CrossRef
96
Jose T., RealSergio, Martínez-HervásM. Carmen, TormosElena, DomenechFederico V., PallardóGuillermo, Sáez-TormoJosep, RedonRafael, CarmenaF. Javier, ChavesJuan F., AscasoAna-Barbara, García-García. (2010) Increased oxidative stress levels and normal antioxidant enzyme activity in circulating mononuclear cells from patients of familial hypercholesterolemia. Metabolism 59, 293-298
CrossRef
97
María Isabel, CovasPhilippe, GambertMontserrat, FitóRafael, de la Torre. (2010) Wine and oxidative stress: Up-to-date evidence of the effects of moderate wine consumption on oxidative damage in humans. Atherosclerosis 208, 297-304
CrossRef
98
Sue Goo, RheeChun-Seok, Cho. Blot-Based Detection of Dehydroalanine-Containing Glutathione Peroxidase with the Use of Biotin-Conjugated Cysteamine. 2010:, 23-34.
CrossRef
99
Andreja, SinkovicDavid, SuranLidija, LokarEva, FliserMojca, SkergetZoran, NovakZeljko, Knez. (2010) Rosemary extracts improve flow-mediated dilatation of the brachial artery and plasma PAI-1 activity in healthy young volunteers. Phytotherapy Research, n/a-n/a
CrossRef
100
Joseph, Loscalzo. (2010) Antioxidant enzyme deficiencies and vascular disease. Expert Review of Endocrinology & Metabolism 5, 15-18
CrossRef
101
Baukje, de RoosJose Miguel, Arbones-MainarGuillermo Rodriguez, Gutiérrez. The Effects of Olive Oils on Hepatic Lipid Metabolism and Antioxidant Defense Mechanisms. 2010:, 887-894.
CrossRef
102
Jane A., LeopoldJoseph, Loscalzo. (2009) Oxidative risk for atherothrombotic cardiovascular disease. Free Radical Biology and Medicine 47, 1673-1706
CrossRef
103
Akihiro, IsogawaMinoru, YamakadoMasao, YanoTeruo, Shiba. (2009) Serum superoxide dismutase activity correlates with the components of metabolic syndrome or carotid artery intima-media thickness. Diabetes Research and Clinical Practice 86, 213-218
CrossRef
104
Lutz, SchomburgUlrich, Schweizer. (2009) Hierarchical regulation of selenoprotein expression and sex-specific effects of selenium. Biochimica et Biophysica Acta (BBA) - General Subjects 1790, 1453-1462
CrossRef
105
Holger, SteinbrennerHelmut, Sies. (2009) Protection against reactive oxygen species by selenoproteins. Biochimica et Biophysica Acta (BBA) - General Subjects 1790, 1478-1485
CrossRef
106
Margaret P., Rayman. (2009) Selenoproteins and human health: Insights from epidemiological data. Biochimica et Biophysica Acta (BBA) - General Subjects 1790, 1533-1540
CrossRef
107
Jun, LuCarsten, BerndtArne, Holmgren. (2009) Metabolism of selenium compounds catalyzed by the mammalian selenoprotein thioredoxin reductase. Biochimica et Biophysica Acta (BBA) - General Subjects 1790, 1513-1519
CrossRef
108
Alison M, HillJennifer A, FlemingPenny M, Kris-Etherton. (2009) The role of diet and nutritional supplements in preventing and treating cardiovascular disease. Current Opinion in Cardiology 24, 433-441
CrossRef
109
G., Flores-MateoP., Carrillo-SantisteveR., ElosuaE., GuallarJ., MarrugatJ., BleysM.-I., Covas. (2009) Antioxidant Enzyme Activity and Coronary Heart Disease: Meta-analyses of Observational Studies. American Journal of Epidemiology 170, 135-147
CrossRef
110
Christian K., RobertsKunal K., Sindhu. (2009) Oxidative stress and metabolic syndrome. Life Sciences 84, 705-712
CrossRef
111
Absalon D., GutierrezDaniela Gonzalez, de SernaIrina, RobinsonDavid S., Schade. (2009) The response of γ vitamin E to varying dosages of α vitamin E plus vitamin C. Metabolism 58, 469-478
CrossRef
112
Reiko, IidaEtsuko, TsubotaIsao, YuasaHaruo, TakeshitaToshihiro, Yasuda. (2009) Simultaneous genotyping of 11 non-synonymous SNPs in the 4 glutathione peroxidase genes using the multiplex single base extension method. Clinica Chimica Acta 402, 79-82
CrossRef
113
Kallol, DuttaBiswadev, Bishayi. (2009) Escherichia coli lipopolysaccharide administration alters antioxidant profile during hypercholesterolemia. Indian Journal of Clinical Biochemistry 24, 179-183
CrossRef
114
Pilar, Codoñer-FranchAmalia, Bataller AlberolaJosé V., Domingo CamarasaMaría C., Escribano MoyaVictoria, Valls Bellés. (2009) Influence of dietary lipids on the erythrocyte antioxidant status of hypercholesterolaemic children. European Journal of Pediatrics 168, 321-327
CrossRef
115
Mei-ling, ChengChin-ming, ChenHung-yao, HoJui-ming, LiDaniel Tsun-yee, Chiu. (2009) Effect of Acute Myocardial Infarction on Erythrocytic Glutathione Peroxidase 1 Activity and Plasma Vitamin E Levels. The American Journal of Cardiology 103, 471-475
CrossRef
116
Cong, LeiXiaolin, NiuJin, WeiJianhong, ZhuYi, Zhu. (2009) Interaction of glutathione peroxidase-1 and selenium in endemic dilated cardiomyopathy. Clinica Chimica Acta 399, 102-108
CrossRef
117
Nadine, PomaredeMeera, Chandramouli. Enhancing the Skin's Natural Antioxidant Enzyme System by the Supplementation or Upregulation of Superoxide Dismutase, Catalase, and Glutathione Peroxidase. 2009:, 245-265.
CrossRef
118
R D, SembaM O, RicksL, FerrucciQ-L, XueJ M, GuralnikL P, Fried. (2009) Low serum selenium is associated with anemia among older adults in the United States. European Journal of Clinical Nutrition 63, 93-99
CrossRef
119
Robyn, LymburyUjang, TinggiLyn, GriffithsFranklin, RosenfeldtAnthony V., Perkins. (2008) Selenium Status of the Australian Population: Effect of Age, Gender and Cardiovascular Disease. Biological Trace Element Research 126, 1-10
CrossRef
120
Renate, SchnabelEdith, LubosClaudia M., MessowChristoph R., SinningTanja, ZellerPhilipp S., WildDirk, PeetzDiane E., HandyThomas, MunzelJoseph, LoscalzoKarl J., LacknerStefan, Blankenberg. (2008) Selenium supplementation improves antioxidant capacity in vitro and in vivo in patients with coronary artery disease. American Heart Journal 156, 1201.e1-1201.e11
CrossRef
121
Lutz, SchomburgJosef, Köhrle. (2008) On the importance of selenium and iodine metabolism for thyroid hormone biosynthesis and human health. Molecular Nutrition & Food Research 52:10.1002/mnfr.v52:11, 1235-1246
CrossRef
122
Yu-I, LiGary, ElmerRenée C., LeBoeuf. (2008) Tanshinone IIA reduces macrophage death induced by hydrogen peroxide by upregulating glutathione peroxidase. Life Sciences 83, 557-562
CrossRef
123
T., KellerC. M., MessowE., LubosV., NicaudP. S., WildH. J., RupprechtC., BickelS., TzikasD., PeetzK. J., LacknerL., TiretT. F., MunzelS., BlankenbergR. B., Schnabel. (2008) Cystatin C and cardiovascular mortality in patients with coronary artery disease and normal or mildly reduced kidney function: results from the AtheroGene study. European Heart Journal 30, 314-320
CrossRef
124
Fares A., MasriSuzy A. A., ComhairIva, Dostanic-LarsonFrancisco Takao, KanekoRaed A., DweikAlejandro C., ArroligaSerpil C., Erzurum. (2008) Deficiency of Lung Antioxidants in Idiopathic Pulmonary Arterial Hypertension. Clinical and Translational Science 1:10.1111/cts.2008.1.issue-2, 99-106
CrossRef
125
Na-Ping, TangLian-Sheng, WangLi, YangHai-Juan, GuQing-Min, SunRi-Hong, CongBo, ZhouHuai-Jun, ZhuBin, Wang. (2008) Genetic variant in glutathione peroxidase 1 gene is associated with an increased risk of coronary artery disease in a Chinese population. Clinica Chimica Acta 395, 89-93
CrossRef
126
Sergio, Martinez-HervasMarta, FandosJose T., RealOlga, EspinosaF. Javier, ChavesGuillermo T., SaezAmparo, SalvadorConcha, CerdáRafael, CarmenaJuan F., Ascaso. (2008) Insulin resistance and oxidative stress in familial combined hyperlipidemia. Atherosclerosis 199, 384-389
CrossRef
127
S., TsimikasJ., WilleitM., KnoflachM., MayrG., EggerM., NotdurfterJ. L., WitztumC. J., WiedermannQ., XuS., Kiechl. (2008) Lipoprotein-associated phospholipase A2 activity, ferritin levels, metabolic syndrome, and 10-year cardiovascular and non-cardiovascular mortality: results from the Bruneck study. European Heart Journal 30, 107-115
CrossRef
128
Burcin, ÖzüyamanMarijke, GrauMalte, KelmMarc W., MerxPetra, Kleinbongard. (2008) RBC NOS: regulatory mechanisms and therapeutic aspects. Trends in Molecular Medicine 14, 314-322
CrossRef
129
Marie-Claire, BélangerMarc-Édouard, MiraultEric, DewaillyLine, BerthiaumePierre, Julien. (2008) Environmental contaminants and redox status of coenzyme Q10 and vitamin E in Inuit from Nunavik. Metabolism 57, 927-933
CrossRef
130
Christian, DellesWilliam H., MillerAnna F., Dominiczak. (2008) Targeting Reactive Oxygen Species in Hypertension. Antioxidants & Redox Signaling 10, 1061-1078
CrossRef
131
Kellan E., AshleyJohn M., GallaStephen J., Nicholls. (2008) Brain Natriuretic Peptides as Biomarkers for Atherosclerosis. Preventive Cardiology 11, 172-176
CrossRef
132
Ulrich, Förstermann. (2008) Oxidative stress in vascular disease: causes, defense mechanisms and potential therapies. Nature Clinical Practice Cardiovascular Medicine 5, 338-349
CrossRef
133
S. E., EspinozaH., GuoN., FedarkoA., DeZernL. P., FriedQ.-L., XueS., LengB., BeamerJ. D., Walston. (2008) Glutathione Peroxidase Enzyme Activity in Aging. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences 63, 505-509
CrossRef
134
Marie-Claire, BélangerMarc-Edouard, MiraultEric, DewaillyMichel, PlanteLine, BerthiaumeMicheline, NoëlPierre, Julien. (2008) Seasonal mercury exposure and oxidant-antioxidant status of James Bay sport fishermen. Metabolism 57, 630-636
CrossRef
135
Fulvio, LauretaniRichard D., SembaStefania, BandinelliAmanda L., RayCarmelinda, RuggieroAntonio, CherubiniJack M., GuralnikLuigi, Ferrucci. (2008) Low plasma selenium concentrations and mortality among older community-dwelling adults: the InCHIANTI Study. Aging Clinical and Experimental Research 20, 153-158
CrossRef
136
Joseph S, MeltzerVivek K, Moitra. (2008) The nutritional and metabolic support of heart failure in the intensive care unit. Current Opinion in Clinical Nutrition and Metabolic Care 11, 140-146
CrossRef
137
Hung-Yao HoPo-Wen, GuYi-Li, WangJui-Ming, LiDaniel Tsun-Yee, ChiuMei-Ling, ChengChin-Ming, Chen. (2008) Oxidative damage markers and antioxidants in patients with acute myocardial infarction and their clinical significance. BioFactors 34:10.1002/biof.v34:2, 135-145
CrossRef
138
Fausta, NatellaMichela, FidaleFranco, TubaroFulvio, UrsiniCristina, Scaccini. (2007) Selenium supplementation prevents the increase in atherogenic electronegative LDL (LDL minus) in the postprandial phase. Nutrition, Metabolism and Cardiovascular Diseases 17, 649-656
CrossRef
139
Tracie L., MillerDaniela, NeriJason, ExteinGabriel, SomarribaNancy, Strickman-Stein. (2007) Nutrition in pediatric cardiomyopathy. Progress in Pediatric Cardiology 24, 59-71
CrossRef
140
Zehra, SerdarDilek, YeşilbursaMelahat, DiricanEmre, SarandölAkın, Serdar. (2007) Sialic acid and oxidizability of lipid and proteins and antioxidant status in patients with coronary artery disease. Cell Biochemistry and Function 25:10.1002/cbf.v25:6, 655-664
CrossRef
141
Edith, LubosJoseph, LoscalzoDiane E., Handy. (2007) Homocysteine and Glutathione Peroxidase-1. Antioxidants & Redox Signaling 9, 1923-1940
CrossRef
142
Mitsutoshi, OguriKimihiko, KatoTakeshi, HibinoKiyoshi, YokoiTomonori, SegawaHitoshi, MatsuoSachiro, WatanabeYoshinori, NozawaToyoaki, MuroharaYoshiji, Yamada. (2007) Genetic risk for restenosis after coronary stenting. Atherosclerosis 194, e172-e178
CrossRef
143
Lutz, SchomburgCornelia, RieseKostja, RenkoUlrich, Schweizer. (2007) Effect of age on sexually dimorphic selenoprotein expression in mice. Biological Chemistry 388, 1035-1041
CrossRef
144
Nisha I, ParikhRamachandran S, Vasan. (2007) Assessing the clinical utility of biomarkers in medicine. Biomarkers in Medicine 1, 419-436
CrossRef
145
John, MercerMelli, MahmoudiMartin, Bennett. (2007) DNA damage, p53, apoptosis and vascular disease. Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis 621, 75-86
CrossRef
146
Ruzena, TkacovaZuzana, KluchovaPavol, JoppaDarina, PetrasovaAngela, Molcanyiova. (2007) Systemic inflammation and systemic oxidative stress in patients with acute exacerbations of COPD. Respiratory Medicine 101, 1670-1676
CrossRef
147
Hong, ZhuZhuoxiao, CaoLi, ZhangMichael A., TrushYunbo, Li. (2007) Glutathione and glutathione-linked enzymes in normal human aortic smooth muscle cells: chemical inducibility and protection against reactive oxygen and nitrogen species-induced injury. Molecular and Cellular Biochemistry 301, 47-59
CrossRef
148
Mia, JülligAnthony J., HickeyMartin J., MiddleditchDavid J., CrossmanStanley C., LeeGarth J. S., Cooper. (2007) Characterization of proteomic changes in cardiac mitochondria in streptozotocin-diabetic rats using iTRAQ™ isobaric tags. PROTEOMICS – Clinical Applications 1:10.1002/prca.v1:6, 565-576
CrossRef
149
Mika J., ThomsonValentina, PuntmannJuan-Carlos, Kaski. (2007) Atherosclerosis and Oxidant Stress: The End of the Road for Antioxidant Vitamin Treatment?. Cardiovascular Drugs and Therapy 21, 195-210
CrossRef
150
Jonica, CampoloRenata, De MariaRaffaele, CarusoRoberto, AccinniFabio, TurazzaMarina, ParoliniElèna, RoubinaBenedetta, De ChiaraGiuliana, CighettiMaria, FrigerioEttore, VitaliOberdan, Parodi. (2007) Blood glutathione as independent marker of lipid peroxidation in heart failure. International Journal of Cardiology 117, 45-50
CrossRef
151
Rudolf, LampéMárk, OrmosSándor, SzűcsRóza, ÁdányEdit, SzikszayRobert, Póka. (2007) Superoxide anion production of granulocytes in patients with endometrial cancer at presentation and after treatment. European Journal of Obstetrics & Gynecology and Reproductive Biology 131, 231-234
CrossRef
152
Christine, Espinola-KleinHans J., RupprechtChristoph, BickelRenate, SchnabelSabine, Genth-ZotzMicheal, TorzewskiKarl, LacknerThomas, MunzelStefan, Blankenberg. (2007) Glutathione Peroxidase-1 Activity, Atherosclerotic Burden, and Cardiovascular Prognosis. The American Journal of Cardiology 99, 808-812
CrossRef
153
Regina, EbertFranz, Jakob. (2007) Selenium deficiency as a putative risk factor for osteoporosis. International Congress Series 1297, 158-164
CrossRef
154
Benedetta, De ChiaraAntonio, MafriciJonica, CampoloGabriella, FamosoValentina, SeddaMarina, ParoliniGiuliana, CighettiAlessandro, LualdiCesare, FiorentiniOberdan, Parodi. (2007) Low plasma glutathione levels after reperfused acute myocardial infarction are associated with late cardiac events. Coronary Artery Disease 18, 77-82
CrossRef
155
N, AbdillaM C, TormoM J, FabiaF J, ChavesG, SaezJ, Redon. (2007) Impact of the components of metabolic syndrome on oxidative stress and enzymatic antioxidant activity in essential hypertension. Journal of Human Hypertension 21, 68-75
CrossRef
156
Rachel, ElsomPeter, SandersonJohn E., HeskethMalcolm J., JacksonSusan J., Fairweather-TaitBjörn, ÅkessonJean, HandyJohn R., Arthur. (2006) Functional markers of selenium status: UK Food Standards Agency workshop report. British Journal of Nutrition 96
CrossRef
157
Christian, DellesAnna F, Dominiczak. (2006) Vascular failure or sick vessel syndrome: the cardiovascular continuum is a useful concept for clinical research. Journal of Hypertension 24, 2147-2148
CrossRef
158
A C, WhiteA M, SousaJ, BlumbergH F, RyanB L, FanburgU S, Kayyali. (2006) Plasma antioxidants in subjects before hematopoietic stem cell transplantation. Bone Marrow Transplantation 38, 513-520
CrossRef
159
María-Isabel, CovasValentina, Ruiz-GutiérrezRafael, TorreAnthony, KafatosRosa M., Lamuela-RaventósJesus, OsadaRobert W., OwenFrancesco, Visioli. (2006) Minor Components of Olive Oil: Evidence to Date of Health Benefits in Humans. Nutrition Reviews 64:10.1111/nure.2006.64.issue-s4, S20-S30
CrossRef
160
Mehmet, AslanMehmet, HorozAbdurrahim, KocyigitSaadet, OzgonülHakim, CelikMetin, CelikOzcan, Erel. (2006) Lymphocyte DNA damage and oxidative stress in patients with iron deficiency anemia. Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis 601, 144-149
CrossRef
161
Matthew A., RobertsDavid L., HareSujiva, RatnaikeFrancesco L., Ierino. (2006) Cardiovascular Biomarkers in CKD: Pathophysiology and Implications for Clinical Management of Cardiac Disease. American Journal of Kidney Diseases 48, 341-360
CrossRef
162
Zehra, SerdarKemal, AslanMelahat, DiricanEmre, SarandölDilek, YeşilbursaAkýn, Serdar. (2006) Lipid and protein oxidation and antioxidant status in patients with angiographically proven coronary artery disease. Clinical Biochemistry 39, 794-803
CrossRef
163
Marie-Claire, BélangerÉric, DewaillyLine, BerthiaumeMicheline, NoëlJean, BergeronMarc-Édouard, MiraultPierre, Julien. (2006) Dietary contaminants and oxidative stress in Inuit of Nunavik. Metabolism 55, 989-995
CrossRef
164
Roland, Gärtner. (2006) Die medizinische Bedeutung von Selen / The clinical relevance of selenium. LaboratoriumsMedizin 30, 201-208
CrossRef
165
Renu, MisraSonika, MangiSujata, JoshiSuneeta, MittalSuresh K., GuptaRavindra M., Pandey. (2006) LycoRed as an alternative to hormone replacement therapy in lowering serum lipids and oxidative stress markers: A randomized controlled clinical trial. Journal of Obstetrics and Gynaecology Research 32, 299-304
CrossRef
166
Enzo, EmanueleColomba, FalconeAngela, D’AngeloPiercarlo, MinorettiMaria P., BuzziMarco, BertonaDiego, Geroldi. (2006) Association of plasma eotaxin levels with the presence and extent of angiographic coronary artery disease. Atherosclerosis 186, 140-145
CrossRef
167
Nageswara R., MadamanchiIgor, TchivilevMarschall S., Runge. (2006) Genetic markers of oxidative stress and coronary atherosclerosis. Current Atherosclerosis Reports 8, 177-183
CrossRef
168
Tetsuya, MatobaBradford, Berk. Inflammation, cardiovascular disease and hypertension. 2006:, 335-342.
CrossRef
169
Salman, AshfaqJerome L., AbramsonDean P., JonesSteven D., RhodesWilliam S., WeintraubW. Craig, HooperViola, VaccarinoDavid G., HarrisonArshed A., Quyyumi. (2006) The Relationship Between Plasma Levels of Oxidized and Reduced Thiols and Early Atherosclerosis in Healthy Adults. Journal of the American College of Cardiology 47, 1005-1011
CrossRef
170
Michel, LorgerilPatricia, Salen. (2006) Selenium and antioxidant defenses as major mediators in the development of chronic heart failure. Heart Failure Reviews 11, 13-17
CrossRef
171
Renate, SchnabelEdith, LubosHans J., RupprechtChristine, Espinola-KleinChristoph, BickelKarl J., LacknerFrançois, CambienLaurence, TiretThomas, MünzelStefan, Blankenberg. (2006) B-Type Natriuretic Peptide and the Risk of Cardiovascular Events and Death in Patients With Stable Angina. Journal of the American College of Cardiology 47, 552-558
CrossRef
172
Emina, ColakNada, Majkic-SinghSanja, StankovicPredrag, DjordjevicVesna, Dimitrijevic-SreckovicKatarina, LalicNebojsa, Lalic. (2006) The effect of hyperglycemia on the values of antioxidative parameters in type 2 diabetic patients with cardiovascular complications. Jugoslovenska medicinska biohemija 25, 173-179
CrossRef
173
Sven, WassmannKerstin, WassmannGeorg, Nickenig. (2006) Regulation of antioxidant and oxidant enzymes in vascular cells and implications for vascular disease. Current Hypertension Reports 8, 69-78
CrossRef
174
Gwo-Hwa, WanKen-Kuo, LinShu-Chen, TsaiDaniel Tsun-Yee, Chiu. (2006) Decreased Glucose-6-PhosphateDehydrogenase (G6PD) Activity and Risk of Senile Cataract in Taiwan. Ophthalmic Epidemiology 13, 109-114
CrossRef
175
L.A., LexisA., FenningL., BrownR.G., FassettJ.S., Coombes. (2006) Antioxidant Supplementation Enhances Erythrocyte Antioxidant Status and Attenuates Cyclosporine-Induced Vascular Dysfunction. American Journal of Transplantation 6:10.1111/ajt.2006.6.issue-1, 41-49
CrossRef
176
Werner , Nikos Kosiol , Sonja Schiegl , Tobias Ahlers , Patrick Walenta , Katrin Link , Andreas Böhm , Michael Nickenig , Georg . (2005) Circulating Endothelial Progenitor Cells and Cardiovascular Outcomes. New England Journal of Medicine 353:10, 999-1007
Free Full Text
177
J.-F., LagabrielleN., DelarcheT.H., AdamouE., Fabre. (2005) Dosage de l'albumine modifiée par l'ischémie (IMA) dans le cadre d'angioplasties programmées. Immuno-analyse & Biologie Spécialisée 20, 221-227
CrossRef
178
Colin D., Funk. (2005) Leukotriene modifiers as potential therapeutics for cardiovascular disease. Nature Reviews Drug Discovery 4, 664-672
CrossRef
179
M., FitóM., CladellasR., de la TorreJ., MartíM., AlcántaraM., Pujadas-BastardesJ., MarrugatJ., BrugueraM.C., López-SabaterJ., VilaM.I., Covas. (2005) Antioxidant effect of virgin olive oil in patients with stable coronary heart disease: a randomized, crossover, controlled, clinical trial. Atherosclerosis 181, 149-158
CrossRef
180
Ira, Tager. Chronic Exposure and Susceptibility to Oxidant Air Pollutants. 2005:, 259-301.
CrossRef
181
Renate, SchnabelKarl J., LacknerHans J., RupprechtChristine, Espinola-KleinMichael, TorzewskiEdith, LubosChristoph, BickelFrançois, CambienLaurence, TiretThomas, MünzelStefan, Blankenberg. (2005) Glutathione Peroxidase-1 and Homocysteine for Cardiovascular Risk Prediction. Journal of the American College of Cardiology 45, 1631-1637
CrossRef
182
Emel, AltekinCanan, ÇokerAli Rıza, ŞişmanBanu, ÖnvuralFiliz, KuralayÖnder, Kırımlı. (2005) The relationship between trace elements and cardiac markers in acute coronary syndromes. Journal of Trace Elements in Medicine and Biology 18, 235-242
CrossRef
183
Guilherme H. M., Oliveira. (2005) Novel serologic markers of cardiovascular risk. Current Atherosclerosis Reports 7, 148-154
CrossRef
184
T., SzelesteiS., BahringZ., WagnerA., AydinG. A., MolnarB., KocsisJ., NagyI., Wittmann. (2005) Serum levels of l-arginine analogues and glutathione peroxidase and catalase gene variants in Type 2 diabetes mellitus patients. Diabetic Medicine 22:10.1111/dme.2005.22.issue-3, 356-357
CrossRef
185
Iftikhar J., KulloChristie M., Ballantyne. (2005) Conditional Risk Factors for Atherosclerosis. Mayo Clinic Proceedings 80, 219-230
CrossRef
186
Mei-ling, ChengHung-yao, HoHsiu-chuan, TsengChien-Hong, LeeLee-yung, ShihDaniel Tsun-yee, Chiu. (2005) Antioxidant deficit and enhanced susceptibility to oxidative damage in individuals with different forms of
α
-thalassaemia. British Journal of Haematology 128:10.1111/bjh.2005.128.issue-1, 119-127
CrossRef
187
José A., PáramoRamón, LecumberriJosune, Orbe. (2005) Trombosis arterial y polimorfismos genéticos: demasiados actores, escenario complejo. Medicina Clínica 124, 69-74
CrossRef
188
Barbara, VoetschRichard C., JinJoseph, Loscalzo. (2004) Nitric oxide insufficiency and atherothrombosis. Histochemistry and Cell Biology 122, 353-367
CrossRef
189
Jesse, Adams. (2004) Markers to define ischemia: Are they ready for prime time use in patients with acute coronary syndromes?. Current Cardiology Reports 6, 253-258
CrossRef
190
Fong-Fong, ChuR.Steven, EsworthyJames H., Doroshow. (2004) Role of Se-dependent glutathione peroxidases in gastrointestinal inflammation and cancer. Free Radical Biology and Medicine 36, 1481-1495
CrossRef
191
Barbara, VoetschJoseph, Loscalzo. (2004) Genetics of thrombophilia: impact on atherogenesis. Current Opinion in Lipidology 15, 129-143
CrossRef
192
Ren?? L, Chiol??roKenneth, Kudsk. (2004) Current concepts in nutrition delivery in critically ill patients: route, insulin, and economics. Current Opinion in Clinical Nutrition and Metabolic Care 7, 157-159
CrossRef
193
Doron, Aronson. (2004) Inflammatory markers: linking unstable plaques to coronary event, an interventional perspective. Acute Cardiac Care 6, 110-118
CrossRef
194
Jagat, NarulaH. William, Strauss. (2004) Imaging of unstable atherosclerotic lesions. European Journal of Nuclear Medicine and Molecular Imaging 32:1, 1
CrossRef
195
Manolio , Teri . (2003) Novel Risk Markers and Clinical Practice. New England Journal of Medicine 349:17, 1587-1589
Full Text
 Base-Line Characteristics of the Study Patients. gives the base-line characteristics of the 83 study participants who subsequently died from cardiovascular causes or had a nonfatal myocardial infarction and the 553 who did not have a cardiovascular event, including the results of base-line blood-chemistry analysis. Glutathione peroxidase 1 activity was normally distributed among the study participants. It ranged from 7.4 to 99.6 units per gram of hemoglobin, with a mean (±SD) of 49.2±11.6, a median of 48.3, and an interquartile range of 42.0 to 56.3 units per gram of hemoglobin. The base-line level of glutathione peroxidase 1 activity was significantly lower among those who died from cardiac causes or had a nonfatal myocardial infarction than among those who did not. This result was stable when data from the subgroups with fatal events (64 patients) and nonfatal events (19 patients) were analyzed separately (45.7±13.5 and 44.1±10.4 units per gram of hemoglobin, respectively, vs. 49.8±11.3 units per gram of hemoglobin for those who did not have cardiovascular events or die from noncardiovascular causes; P=0.009 and P=0.03, respectively). The base-line level of glutathione peroxidase 1 activity in patients who died from noncardiovascular causes (21 patients) was the same as that in event-free patients (49.7±11.1 vs. 49.8±11.3 units per gram of hemoglobin).
Base-Line Characteristics of the Study Patients. gives the base-line characteristics of the 83 study participants who subsequently died from cardiovascular causes or had a nonfatal myocardial infarction and the 553 who did not have a cardiovascular event, including the results of base-line blood-chemistry analysis. Glutathione peroxidase 1 activity was normally distributed among the study participants. It ranged from 7.4 to 99.6 units per gram of hemoglobin, with a mean (±SD) of 49.2±11.6, a median of 48.3, and an interquartile range of 42.0 to 56.3 units per gram of hemoglobin. The base-line level of glutathione peroxidase 1 activity was significantly lower among those who died from cardiac causes or had a nonfatal myocardial infarction than among those who did not. This result was stable when data from the subgroups with fatal events (64 patients) and nonfatal events (19 patients) were analyzed separately (45.7±13.5 and 44.1±10.4 units per gram of hemoglobin, respectively, vs. 49.8±11.3 units per gram of hemoglobin for those who did not have cardiovascular events or die from noncardiovascular causes; P=0.009 and P=0.03, respectively). The base-line level of glutathione peroxidase 1 activity in patients who died from noncardiovascular causes (21 patients) was the same as that in event-free patients (49.7±11.1 vs. 49.8±11.3 units per gram of hemoglobin).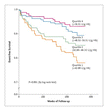 Kaplan–Meier Curves Showing Cardiovascular Events According to Quartile of Glutathione Peroxidase 1 Activity. shows the Kaplan–Meier curves for event-free survival according to quartile of glutathione peroxidase 1 activity. The unadjusted rate of cardiovascular events increased in a stepwise fashion across decreasing quartiles of base-line glutathione peroxidase 1 activity. The difference between the lowest and highest quartiles and the trend across all quartiles were significant (P<0.001 for both comparisons). The event rate for patients in the lowest quartile of glutathione peroxidase 1 activity (20.8 percent) was approximately three times that for patients in the highest quartile (7.0 percent). To place the effect in perspective, Table 2Table 2
Kaplan–Meier Curves Showing Cardiovascular Events According to Quartile of Glutathione Peroxidase 1 Activity. shows the Kaplan–Meier curves for event-free survival according to quartile of glutathione peroxidase 1 activity. The unadjusted rate of cardiovascular events increased in a stepwise fashion across decreasing quartiles of base-line glutathione peroxidase 1 activity. The difference between the lowest and highest quartiles and the trend across all quartiles were significant (P<0.001 for both comparisons). The event rate for patients in the lowest quartile of glutathione peroxidase 1 activity (20.8 percent) was approximately three times that for patients in the highest quartile (7.0 percent). To place the effect in perspective, Table 2Table 2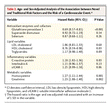 Age- and Sex-Adjusted Analysis of the Association between Novel and Traditional Risk Factors and the Risk of a Cardiovascular Event. presents the hazard ratios for cardiovascular events associated with an increase of 1 SD in various risk factors.
Age- and Sex-Adjusted Analysis of the Association between Novel and Traditional Risk Factors and the Risk of a Cardiovascular Event. presents the hazard ratios for cardiovascular events associated with an increase of 1 SD in various risk factors.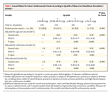 Hazard Ratios for Future Cardiovascular Events According to Quartile of Base-Line Glutathione Peroxidase 1 Activity.). The inverse relation between glutathione peroxidase 1 activity and relative risk remained nearly unchanged after adjustment for cardiovascular risk factors and clinical features (model 2). Further adjustment for therapeutic variables as well as C-reactive protein (as a cluster representative of the inflammatory markers), homocysteine, and creatinine (model 3) also did not attenuate the relative risk associated with glutathione peroxidase 1 activity; patients in the highest quartile of glutathione peroxidase 1 activity had a hazard ratio of 0.29 (95 percent confidence interval, 0.14 to 0.60; P=0.001) as compared with those in the lowest quartile. Inclusion of interleukin-6 instead of C-reactive protein in the Cox predictive model had no effect on the hazard ratios. Similarly, in a subgroup of 566 patients in whom the ejection fraction had been measured, adjustment for this variable did not alter the hazard ratio associated with increasing quartiles of glutathione peroxidase 1 activity (data not shown).
Hazard Ratios for Future Cardiovascular Events According to Quartile of Base-Line Glutathione Peroxidase 1 Activity.). The inverse relation between glutathione peroxidase 1 activity and relative risk remained nearly unchanged after adjustment for cardiovascular risk factors and clinical features (model 2). Further adjustment for therapeutic variables as well as C-reactive protein (as a cluster representative of the inflammatory markers), homocysteine, and creatinine (model 3) also did not attenuate the relative risk associated with glutathione peroxidase 1 activity; patients in the highest quartile of glutathione peroxidase 1 activity had a hazard ratio of 0.29 (95 percent confidence interval, 0.14 to 0.60; P=0.001) as compared with those in the lowest quartile. Inclusion of interleukin-6 instead of C-reactive protein in the Cox predictive model had no effect on the hazard ratios. Similarly, in a subgroup of 566 patients in whom the ejection fraction had been measured, adjustment for this variable did not alter the hazard ratio associated with increasing quartiles of glutathione peroxidase 1 activity (data not shown).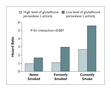 Age- and Sex-Adjusted Hazard Ratios for Cardiovascular Events According to the Level of Glutathione Peroxidase 1 Activity and Smoking Status., among those with low levels of glutathione peroxidase 1 activity (at or below the median value of 48.32 units per gram of hemoglobin), former smokers were at significant risk for cardiovascular events (hazard ratio, 3.0, as compared with patients who had never smoked and had high glutathione peroxidase 1 activity; 95 percent confidence interval, 1.5 to 5.9; P=0.001), as were current smokers (hazard ratio, 5.6; 95 percent confidence interval, 2.4 to 13.5; P<0.001). To a lesser extent, a significant increase in cardiovascular risk was also observed in former or current smokers in the subgroup of those with levels of glutathione peroxidase 1 activity above the median.
Age- and Sex-Adjusted Hazard Ratios for Cardiovascular Events According to the Level of Glutathione Peroxidase 1 Activity and Smoking Status., among those with low levels of glutathione peroxidase 1 activity (at or below the median value of 48.32 units per gram of hemoglobin), former smokers were at significant risk for cardiovascular events (hazard ratio, 3.0, as compared with patients who had never smoked and had high glutathione peroxidase 1 activity; 95 percent confidence interval, 1.5 to 5.9; P=0.001), as were current smokers (hazard ratio, 5.6; 95 percent confidence interval, 2.4 to 13.5; P<0.001). To a lesser extent, a significant increase in cardiovascular risk was also observed in former or current smokers in the subgroup of those with levels of glutathione peroxidase 1 activity above the median.









