BioWeb
Glossary of biochemical terms
Enzyme Properties
I proteins I catalyst I activation energy I
| As
Proteins:
As proteins, enzymes possess
properties in common with other proteins. They are large, with
molecular
weights from 10,000 to well over a million. For instance,
ribonuclease
has a molecular weight of 12,700. Enzymes are labile (unstable).
Thus they may suffer subtle changes in their structure, called
denaturation.
This in turn can cause them to lose activity. Hence, they have to
be handled carefully. Adverse conditions of temperature, pH and
salt
concentration are some of the factors that cause inactivation.
All enzymes are polyelectrolytes i.e., they contain many ionizable groups. Amino acids components are ionizable: such as carboxyl, and basic amine. Consider amino acids such as Asp (asparagine), Glu (glutamine), His (histidine), Arg (arginine), and Lys (lysine). As the pH changes, these groups become more or less ionized, so the net charge on the enzyme depends upon pH. At low pH, the charge is positive, while at alkaline pH, it is negative. While the charge on the entire molecule is important, it is particularly crucial that the catalytic site bears the correct charge. [Check out Dr. Kirk McMichael's discussion of this on his Amino Acid Structure Page, with diagrams: from Washington State University] |
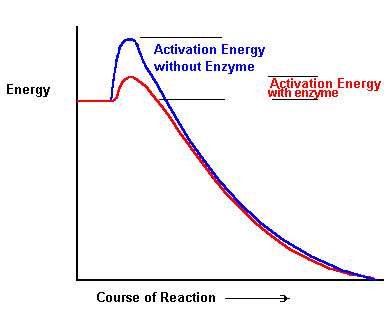
| The
Basis of catalysis: Consider
a very simple reaction such as: S S may conceivably be a substance which can be kept in the laboratory, apparently unchanged, for long periods of time. For the reaction S [Take a look at the diagram to the right. For the original and an explanation see Dr. Robert J. Huskey's Activation Energy and Enzymes I: University of Virginia] |
 |
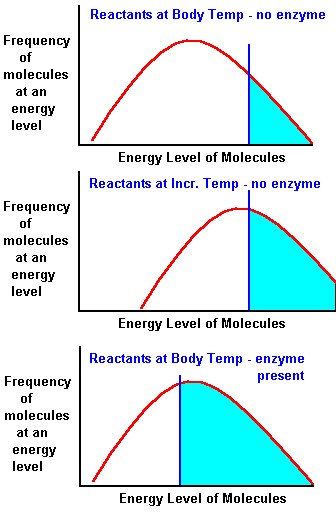
| If
the reactions
require heat why Don't we Just Apply Heat in the Body?
It Would Help (see middle graph to the right)! But neither we nor the enzymes are very stable at high temperatures. In fact most vertebrate enzymes are denatured at temperatures above 40oc. This denaturation (inactivation of the enzyme) is a result of breaking of H bonds and some electrostatic interactions. These bonds are very important in maintaining the active structure (conformation) of the enzyme. It Turns Out That Enzymes
are More Efficient, even WITHOUT added
Heat!
|
 |