Human Learning and Memory
Alzheimer’s disease
Alzheimer’s disease is the most common form of dementia, progressive cognitive decline due to accumulating brain pathology. Over 5 million Americans currently have the disease. The common, late-onset form of Alzheimer’s strikes people aged 60+; the rare early-onset form can strike people as young as their 30s. With a rapidly aging population, the incidence of Alzheimer’s is expected to increase in the coming decades, unless a cure or means of prevention is found. Right now, the underlying causes of late-onset Alzheimer’s are unknown, although some genes and lifestyle factors have been shown to increase an individual’s risk of developing the disease.
Many researchers now believe that the brain pathology underlying Alzheimer’s (the formation of “plaques” and “tangles” in the brain) starts years or even decades before an individual begins to show symptoms of the disease, such as extreme forgetfulness. A key research challenge is therefore to detect this pathology before symptoms appear, providing clinicians with a time window to treat or reverse the damage; early detection would also facilitate drug discovery by allowing clinical researchers to identify individuals who are most likely to benefit from new medications to protect against Alzheimer’s.
Several new techniques have been developed to detect early Alzheimer’s-related pathology; among these is structural neuroimaging (MRI) of the hippocampus and associated brain structures; individuals who show atrophy or shrinkage of these structures are at high risk to show cognitive decline and/or develop Alzheimer’s symptoms over the next 1-2 years. However, right now MRI is costly as a screening method; a cheaper screening approach might be to seek behavioral evidence of hippocampal dysfunction – which might in turn be a marker for hippocampal atrophy.
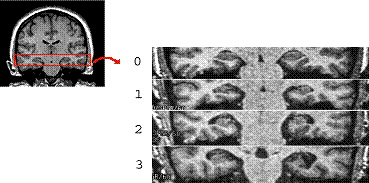
Hippocampal atrophy, visible on structural neuroimaging (MRI) can predict an elderly individual’s short-term risk of Alzheimer’s disease. Here, sections from four individuals are shown, ranging from 0=no visible atrophy through 3=severe atrophy. Adapted from Myers et al. (2003), Journal of Cognitive Neuroscience, 15(2), 185-193.
SMBI researchers and colleagues have developed a battery of simple, computer-based learning and memory tests, which are sensitive to hippocampal function. Many of these involve first training the individual to make a particular keypress in response to each of several stimuli on the screen, and then challenge the individual to transfer this learning when familiar stimuli are altered or presented in new recombinations. Individuals with hippocampal damage tend to be able to complete the initial learning, but are impaired at the subsequent transfer test. Several studies, conducted in collaboration with the NYU Alzheimer’s Disease Center, have now documented that non-demented elderly individuals with hippocampal atrophy show significant disruption on hippocampal-dependent transfer tests – even though they do not (yet) show symptoms of Alzheimer’s. A follow-up longitudinal study on a small group of non-demented elderly has shown that poor performance on a hippocampal-dependent transfer test is associated with increased risk of cognitive decline over the next two years, suggesting that hippocampal-dependent transfer tests might be sensitive indicators of impending cognitive decline and risk for Alzheimer’s.
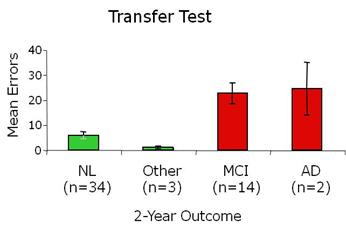 Results from a small-scale longitudinal prediction study show that non-demented elderly individuals who are cognitively normal (NL) at two-year follow-up tend to score well at initial assessment on a hippocampal-dependent transfer test; individuals who have developed mild cognitive impairment (MCI) or Alzheimer’s (AD) at two-year follow-up tend to score poorly at initial testing. Adapted from Myers et al. (2008) Journal of Geriatric Psychiatry and Neurology, 21, 93-103.
Results from a small-scale longitudinal prediction study show that non-demented elderly individuals who are cognitively normal (NL) at two-year follow-up tend to score well at initial assessment on a hippocampal-dependent transfer test; individuals who have developed mild cognitive impairment (MCI) or Alzheimer’s (AD) at two-year follow-up tend to score poorly at initial testing. Adapted from Myers et al. (2008) Journal of Geriatric Psychiatry and Neurology, 21, 93-103.
A long-term goal of this research is to develop and validate such simple behavioral tests for use as general-purpose screening tools that could be used to assess an individual’s short-term risk for Alzheimer’s, to be used alone or in combination with other predictors such as MRI and genetic testing.
Representative Publications:
Myers, C. E., et al. (2008). Learning and generalization tasks predict short-term cognitive outcome in non-demented elderly. Journal of Geriatric Psychiatry and Neurology, 21, 93-103. Hyperlink to abstract: PMID: 18474718
Gluck, M., Myers, C., et al. (2006). Computational models of the hippocampal region: Implications for the prediction of risk for Alzheimer’s disease in non-demented elderly. Current Alzheimer’s Research, 3(3), 247-257. Hyperlink to free article: http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1626443/?tool=pubmed
Myers, C., et al. (2003). Dissociating hippocampal vs. basal ganglia contributions to learning and transfer. Journal of Cognitive Neuroscience, 15(2), 185-193. Hyperlink to abstract: PMID: 12676056
Bódi, N., et al. (2009). Associative learning, acquired equivalence, and flexible generalization of knowledge in mild Alzheimer’s disease. Cognitive and Behavioral Neurology, 22(2):89-94. Hyperlink to abstract: PMID: 19506424
Prior Funding: Institute for the Study of Aging, Fidelity Foundation, Alzheimer’s Association
