|
Amino
Acids
| Peptide formation | Side Chains | Covalent
Modification | Optical Rotation | Amino Acids Grouped |
The
amino acids are the monomeric units from which proteins are
derived. The word protein is derived from the (Greek) word
proteios which means principal or prime. Proteins are, in fact,
principal components of biochemical systems. They serve in a
structural capacity, they are utilized as a source of energy, and they
can be catalysts - most enzymes that catalyze the reactions occurring
in living organisms are proteins. The characteristics the amino acids are important to
the structure and functions of the polymers, the proteins.
The
most commonly occurring amino acids are shown in the table
below. They are usually characterized on the basis of the fourth
substituent (i.e., that in addition to the amino group, the carboxyl
group, and the hydrogen) that is bonded at C (2). The trivial name of
the amino acid is followed by its abbreviation in parentheses.
Next, the systematic name of the amino acid is given. Common structure
of amino acids is:
R--CH--COOH
|
NH2
|
In this structure the C in -COOH
is "carbon #1" and is attached to a -CH-, which is called the "alpha" carbon or "carbon atom
#2", or C(2).
The amino group attached
to alpha carbon is known as "alpha amino group". The R group attached to alpha carbon is known
as side chain. |
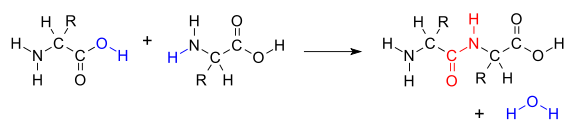
Peptide Formation
The formation
of a protein from amino acids entails a polymerization process
involving the amino group of one amino acid and the carboxyl group of
another, with the release of water and the formation of a perptide bond.
 |
Practice
Exam on the Proteins Page (click on the
"practice exam" on the left, bottom), from Seager/Slabagh's Chemistry
Today [check out the additional information, too.] |
Importance of side chains
The side chain
bonded at C(2) is not involved in forming the peptide bonds which
create the protein. But they may
be available to participate in the reactions and
processes in which the protein is involved. For example, they may
help form hydrogen bonds, or electrostatic and hydrophobic
interactions, or disulfide bonds.,
undergo covalent modification (phosphorylation,
methylation, adenylylation) that alter the chemical or physical
characteristics of the protein,
act as proton donors or proton acceptors in a reaction
mechanism when the protein is an enzyme,
influence the conformation of a
structural element and, thereby, alter the nature of its
contribution to the structural characteristics of the molecule (this is
where secondary and tertiary proptein structure may be developed).
Covalent Modification
Certain functional groups at C(2) of an aminoacyl group undergo enzymatic covalent
modification for the purpose of altering the behavior of the
protein. Such covalent modification is one of the principal ways
in which the catalytic activity of regulatory enzymes is
modulated.
One of the most common types of covalent
modification for the purpose of regulation is phosphorylation of the
hydroxyl group of a serine, or a threonine, or a tyrosine unit of a
protein. This is one example in which the presence of a
side-chain functional group of an amino acid makes it possible for the
protein containing that amino acid to have an important role in
regulating such fundamental processes as cell development or cell
proliferation.
Optical Rotation
All
amino acids except glycine rotate the plane of polarized light because
of the presence of an asymetric center at C(2). The definition of D and L depends on the position of
-NH2 group on C(2).
|
COOH
|
H - C - NH2
|
R
|
This is the D-form.
When the amino group is on the left of C(2), it is called L
form. |
Grouping of amino acids
[see table below]
| Acidic and Basic |
Hydrophilic and Hydrophobic |
Zwitter Ions |
|
At
pH 7, the amino acids are grouped as:
Acidic
(negatively charged): Glutamic acid (Glu) and Aspartic acid
(Asp)
Basic
(positively charged): Lysine (Lys), Arginine (Arg), Histidine
(His)
Neutral:
Rest of the amino acids have net zero charge at neutral pH.
The charge depends on the side chain.
|
Hydrophilic: Ala, Arg,
Asn (Asparagine), Asp, Cys, Glu, Gln (Glutamine), Gly,His, Lys, Pro,
Ser, Thr. Sometimes Tyr is included in this group though Tyr is more
hydrophobic than hydrophilic.
Hydrophobic:
Amino acid side chains other than those above are hydrophobic.
This property depends on whether the side chains like water
(hydrophilic) or hate water (hydrophobic).
|
Any
compound which has a net zero charge is called a zwitter ion. Among
amino acids, neutral amino acids have net zero charge at pH 7
because their structure at this pH is:
R-CH-COO-
|
N+H3
The positive and negative charges
neutralize each other.
|
Amino Acid Table
| Amino acids in
orange have
hydrophobic side chain R groups. Amino acids in green
are considered to be hydrophilic because they have electronegative
groups on the side chain except tyrosine which because of the phenyl
ring side chain is also hydrophobic in character. Two amino acids in pink, Glu and Asp, have two carboxylic
acids in the side chain, are hydrophilic and contribute one negative
charge to a polypeptide chain at neutral pH. The basic amino acids in light blue are also very hydrophilic
and are positively charged at neutral pH. It should be clear from this
that amino acid side chains which contribute to overall charge on a
protein are either acidic or basic at neutral pH. |
The structure of amino acids
shown here are by Dr. Robert J. Huskey (retired) University
of Viginia. 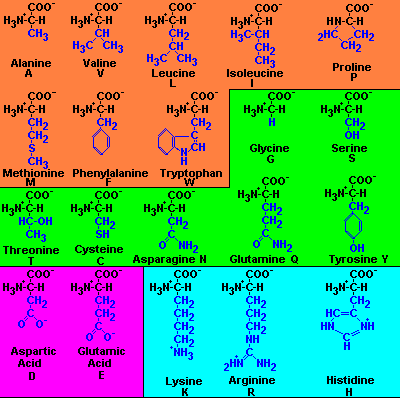
|
|