
Chemical Bonds
| Covalent | Hydrogen | Ionic | Hydrophobic |
|
Covalent
Bonds: These are the type of
bonds formed by two atoms sharing a pair of electrons. Covalent bonds
are what lead to a primary structure of a molecule. These are high
energy bonds with single bond energies of 50-100 Kcal/mole. See more at
Birkbeck
College (University of London). The following table summarizes
covalent bond information of common elements of Biochemistry.
For a description of all types
of bonding see MIT's
Chemistry Review.
|
ELEMENT
|
CHARGE
|
#
COVALENT BONDS
|
EXAMPLE
|
STRUCTURE
|
|
Nitrogen (N)
|
positive
charge on
nitrogen
|
4
|
Ammonia
|
H
|+
H--N--H
|
H
|
|
Oxygen (O)
|
negative charge on
oxygen
|
1
|
ionized ethanol
|
H H
| | _
H--C--C--O
| |
H H
|
|
Sulfur (S)
|
negative charge on the
sulfur
|
1
|
ionized
mercaptoethanol
|
H H
| | -
H--C--C--S
| |
H H
|
Hydrogen
Bonds: In the case of "hydrogen bonds", the terms bonds and
interactions are used interchangeably.
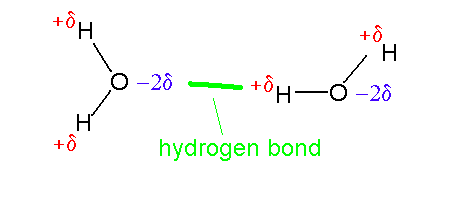
This is a water dimer with H
bond between oxygen of one water and H atom of another molecule. |
Here,
first the H is covalently bonded to an elecronegative atom (such
as an O).
Secondly, the shared electron cloud is shifted
somewhat toward the electronegative element (the O), so leaving a
partial positive charge on H.
Third, the partially positively charged H is able to
interact (form a hydrogen bond) with the partially negatively
charged electronegative atom ( O) of another molecule. |
Hydrogen bonds
(interactions) are weaker than covalent bonds. They have a bond energy
of ~5 Kcal/mole. The bonds are useful iin proteins, enzymes and DNA
because they provide structural stability.
|
The partially positively charged H of
alcohol and the H bond to the N of primary amine.
Note: that R stands for any side
group.
|
H
|
R--O--H ......
N--R
|
H
|
| The
partially positively charged H of an amine and the H bond to the
carbonyl oxygen of a ketone. |
R2--N--H
......
O=CR2 |
|
|
Ionic Bonds
Note: Ionic Bonds or Electrostatic
Interactions are terms that are used interchangeably.
The interaction is
between a negatively charged oxygen atom
of a group like a carboxylate of
glutamate or aspartate and a positively charged
side chain of of lysine, arginine or histidine.
This kind of interaction occurs in proteins at
physiological pH because the carboxylate groups are negatively charged
at pH 7.4 (pKa is ~4.0), and the Arg, Lys, and His side chains are
positively charged.
For additional information, see a review by MIT-Birkbeck. |
|
Hydrophobic
Interactions or Bonds: These interactions occur between
hydrophobic chains present in organic compounds or side chains of
hydrophobic amino acids.
A simple example of hydrophobia is a drop of oil in
water. Oil is not miscible with water because hydrophobic chains of
fatty acids hate water and prefer each other. A vigorous shaking
(providing energy) breaks up the oil droplet into micelles but if you
let them sit for a while, strong hydrophobic interactions will force
the micelles into the original drop.
The hydrophobic bonds are stronger than H bonds but
weaker than covalent bonds.
In a globular protein (which is soluble in water),
hydrophobic side chains tend to point to the interior of the protein
structure and away from bulk water.
On the other hand, hydrophobic side chains of
membrane proteins interact with the membrane's phospholipid membrane
bilayers, and so do not tend to aggregate to the interior.
|
|
|
DEPARTMENT OF
BIOCHEMISTRY AND MOLECULAR BIOLOGY, 185 South Orange Avenue, Newark, NJ 07103-2714.
Phone: 973-972-4750. FAX:
973-972-5594. For information, contact Dr. Kumar: kumarsu@umdnj.edu
|
|